Precision Additive Manufacturingfor Healthcare & Medical Devices
Forge Labs partners with medtech innovators to deliver high-performance prototypes, functional components, and short-run production parts using industrial 3D printing—all under ISO-aligned processes and cleanroom handling capabilities. We support biocompatible materials and traceable manufacturing for regulated medical device production.

Healthcare & Medical Devices Excellence
Supporting Innovation in Medical Manufacturing
Healthcare moves fast—and medical device innovation demands rapid prototyping, precision engineering, and materials that meet strict performance and biocompatibility criteria. At Forge Labs, we help bridge the gap between concept and production with advanced additive manufacturing solutions tailored for the healthcare sector. From ventilator components and surgical prototypes to diagnostic housings and wearable enclosures, our experience spans early-stage design validation to functional parts that meet rigorous inspection standards.
Transforming Healthcare & Medical Devices Manufacturing
Healthcare moves fast—and medical device innovation demands rapid prototyping, precision engineering, and materials that meet strict performance and biocompatibility criteria. At Forge Labs, we help bridge the gap between concept and production with advanced additive manufacturing solutions tailored for the healthcare sector. From ventilator components and surgical prototypes to diagnostic housings and wearable enclosures, our experience spans early-stage design validation to functional parts that meet rigorous inspection standards.
Key Benefits of 3D Printing in Healthcare & Medical Devices
- Dramatically compress design and development cycles from weeks to days, accelerating time-to-market for critical medical innovations.
- Create complex internal geometries optimized for airflow, optical performance, or precise fluid management that are impossible with traditional manufacturing.
- Access a comprehensive range of functional materials including biocompatible, sterilizable, and regulatory-compliant options for medical applications.
- Eliminate expensive tooling investments, enabling cost-effective iterative prototyping and economical short-run production.
- Enable cost-efficient customization and patient-specific solutions without the traditional cost penalties of low-volume manufacturing.
- Leverage ISO 9001-aligned manufacturing processes with cleanroom handling capabilities for medical device compliance.




ISO-Aligned Quality Processes
All medical parts manufactured under rigorous quality inspection aligned with ISO 9001 principles and controlled protocols.
Biocompatible Materials
USP Class VI certified materials with comprehensive biocompatibility testing and documentation for medical device applications.
Cleanroom Handling Capability
Controlled environment processing available for critical medical applications requiring contamination-free manufacturing and handling.
Material Traceability & Documentation
Complete dimensional inspection reports, material certifications, and batch traceability documentation provided upon request.
Manufacturing Capabilities
Advanced Healthcare & Medical Devices Solutions
From precision prototyping to full-scale production, our comprehensive capabilities deliver results that transform healthcare & medical devices manufacturing.
Stereolithography (SLA) with USP Class VI biocompatible resins accelerates medical device development with functional prototypes suitable for clinical evaluation and human factors testing. Tolerances of ±0.25% (min ±0.25 mm) support precise fit requirements for surgical instruments and patient interfaces. High-definition surface finish eliminates post-processing for visualization studies, while multiple biocompatible material options enable testing across device applications. Digital design iteration reduces development timelines from months to days, supporting rapid innovation cycles critical in medical device development.
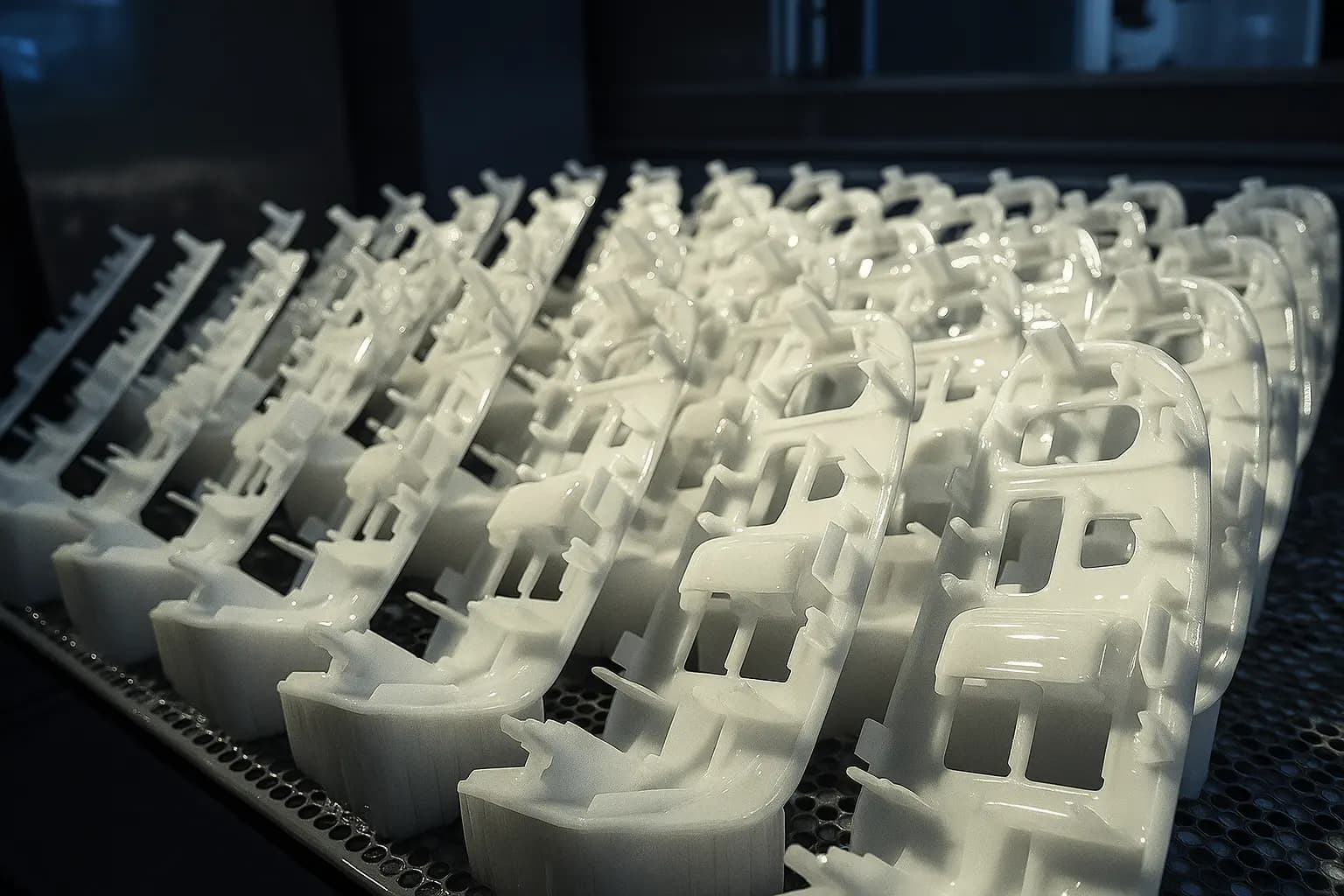
Medical Device Prototyping. SLA Tolerances: ±0.25% (min ±0.25 mm).
Technical Specifications
Accura ClearVue and specialized optical resins provide exceptional transparency and dimensional stability for diagnostic equipment housings, light guides, and optical assemblies. Materials achieve >95% light transmission while maintaining biocompatibility for patient-contact applications. Precision manufacturing ensures critical optical alignment features maintain tolerances throughout sterilization cycles. Custom geometries optimize light path efficiency and minimize optical losses in portable diagnostic devices. Post-processing options include optical polishing and anti-reflective coatings for enhanced performance.

Diagnostic & Imaging Equipment Components. Light Transmission: >95%.
Technical Specifications
Selective laser sintering (SLS) with medical-grade PA12 nylon produces complex respiratory components with smooth internal airflow channels and precise dimensional control. Material properties include chemical resistance to disinfectants, steam sterilization compatibility, and excellent fatigue resistance for cyclic pressure applications. Internal channel geometries optimize laminar airflow while minimizing pressure drops across manifolds and valve assemblies. No support structures required enables complex geometries including integrated filters, flow sensors, and pressure relief features impossible with traditional manufacturing.

Respiratory & Ventilator Components. Channel Tolerance: ±0.3mm.
Technical Specifications
Flexible TPU materials and lightweight nylon enable patient-specific wearable device prototypes with anatomical conformance and comfort optimization. Flexible shore hardness options (70A-95A) accommodate varying compression requirements for monitoring devices, therapeutic equipment, and prosthetic interfaces. Design iteration supports anthropometric optimization across patient populations while maintaining consistent performance characteristics. Biocompatible formulations ensure skin compatibility for extended wear applications in research and clinical validation studies.
Relevant Technologies
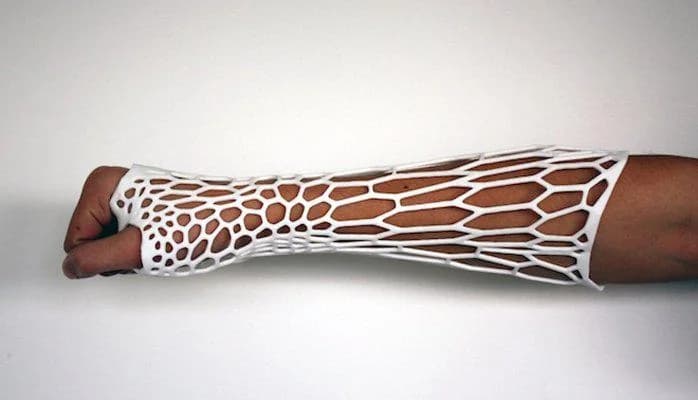
Patient-Specific & Wearable Devices. Shore Hardness: 70A-95A TPU.
Technical Specifications
ISO 9001-aligned quality management systems support cleanroom manufacturing for medical device pilot production and clinical trial components. Environmental controls maintain Class 10,000 (ISO 7) cleanliness standards with documented contamination control procedures. Material handling protocols ensure comprehensive process documentation from raw powder through final packaging with detailed documentation packages. Statistical process control monitoring validates dimensional consistency across production batches while maintaining full chain of custody for regulatory compliance. Cleanroom protocols accommodate volumes from 10 to 10,000+ parts with scalable quality assurance.

Cleanroom Pre-Production Runs. Cleanroom Class: ISO 7 (10,000).
Technical Specifications
High-precision surgical guides and clinical instrumentation manufactured with dimensional accuracies suitable for patient-specific surgical planning and training applications. ULTEM and biocompatible materials withstand repeated sterilization cycles while maintaining dimensional stability and surface quality. Custom patient-specific geometries enable precise surgical placement of implants, optimize access angles, and reduce procedure time. Training models provide realistic tactile feedback with appropriate material properties for skill development and procedural validation in medical education programs.
Relevant Technologies

Surgical & Clinical Tools. Dimensional Accuracy: ±0.25mm.
Technical Specifications
Case Studies
Success Stories
Real results from our healthcare & medical devices partnerships
Ventilator Component Development
Rapid prototyping and production of critical ventilator manifold components during global health crisis. Delivered functional parts with smooth internal airflow channels and biocompatible materials, enabling accelerated device deployment and patient care.

Diagnostic Equipment Housing
Custom optical housing for portable diagnostic device featuring integrated light guides and precise optical alignment features. Achieved optical clarity requirements while maintaining impact resistance for field use applications.

Certified Materials
Healthcare & Medical Devices-Grade Materials
High-performance materials engineered and certified for demanding healthcare & medical devices applications with full traceability and compliance documentation.

Accura ClearVue
Healthcare & Medical Devices grade
Crystal clear biocompatible resin
- Medical device housings
- Excellent detail resolution
- UV-stable formulation

Nylon PA12
Healthcare & Medical Devices grade
Biocompatible engineering grade
- Medical equipment housings
- Chemical resistance
- Sterilization compatible

TPU Medical
Healthcare & Medical Devices grade
Medical grade flexible polymer
- Skin-safe applications
- Excellent biocompatibility
- Soft-touch medical devices

ULTEM 1010
Healthcare & Medical Devices grade
High-performance thermoplastic
- Autoclave sterilizable
- Chemical resistance
- High temperature performance

PC ISO
Healthcare & Medical Devices grade
Medical grade polycarbonate
- Transparent medical devices
- Impact resistant
- USP Class VI certified

ABS M30i
Healthcare & Medical Devices grade
Medical grade ABS
- Biocompatible properties
- Easy post-processing
- Good dimensional stability
Explore Our Complete Material Matrix
Compare properties, applications, and certifications across our entire material portfolio. Filter by technology, industry, or specific requirements.
Manufacturing Technologies
Proven Technologies for Healthcare & Medical Devices
Each technology offers unique advantages for healthcare & medical devices applications. Choose the right process for your specific requirements.
Stereolithography (SLA)
Selective Laser Sintering (SLS)
Multi-Jet Fusion (MJF)
Fused Deposition Modeling (FDM)
Not sure which technology is right for your project?
Our application engineers can help you select the optimal manufacturing process based on your specific requirements, materials, and timeline.
Ready to Transform Your Healthcare & Medical Devices Manufacturing?
Upload your CAD files for an instant quote or speak with our application engineers about your specific requirements.
Click to start your quote
STL, STEP, OBJ, 3MF supported | Get instant pricing